

Cancer is a fatal disease that not only causes health problems but also financial problems. In many places where super-advanced treatments for cancer, such as CAR T-cell therapy, are costly, they are not affordable for most people.
However, what would happen if there was a way to offer these life-saving treatments for a lower price? That's where CAR T-cell therapy in India takes centre stage. This unique way of cancer treatment has been a game-changer for people with some kinds of blood cancer. The most impressive thing is that it is not as expensive as other treatments. In a country like India, where medical expenses can be a worry, making CAR T-cell therapy accessible to more people is a significant step.
The latest approval of NexCAR19, India's own CAR T-cell therapy, is a considerable leap in cancer treatment. The joint effort of Indian scientists and experts from different countries gave birth to NexCAR19, which, in a way, provides cancer patients hope instead of pushing them into bankruptcy. India displays one of the methods of making the treatment affordable to everybody in the country, which is using local technology and skills.
With the coming of NexCAR19, cancer patients in India can start to envisage a future where they can get the treatment they need without spending a lot of money. And India continues to pump investments into new techs, research, and treatment for cancer; the future is bright for millions of people coping with the cruel disease. CAR T-cell therapy is a medical achievement that promises a better and fairer world.

CAR T cell therapy, which is also known as chimeric antigen receptor T-cell therapy, is a groundbreaking way of treating cancer. A patient's T cells are obtained and modified in the laboratory to ensure they can accurately recognise and destroy cancer cells.
This novel treatment is based on an approach where T cells are isolated from the patient’s blood and genetically modified by creating special receptors called chimeric antigen receptors (CARs). The receptors allow the T cells to identify specific proteins on the surface of cancer cells. Thus, the cells can be targeted and destroyed.
CAR T cancer treatment has demonstrated unprecedented results in curing specific types of blood cancers, such as B-cell ALL and certain types of lymphoma. Clinical trials showed effective response rates and some complete disappearances in certain patients who were not responding to standard treatments.
Although it is very effective, CAR T cell therapy is not without risks. Although the treatment can cause serious side effects, such as cytokine release syndrome and neurological toxicity, these complications must be handled by medical professionals with due diligence.
In sum, CAR T cell therapy is noteworthy as it is the breakthrough in cancer treatment and gives hope to patients who have few options for treatment. The ongoing research and clinical trials are investigating its use in the treatment of other types of cancer and the improvement of the outcomes for cancer patients all over the world.
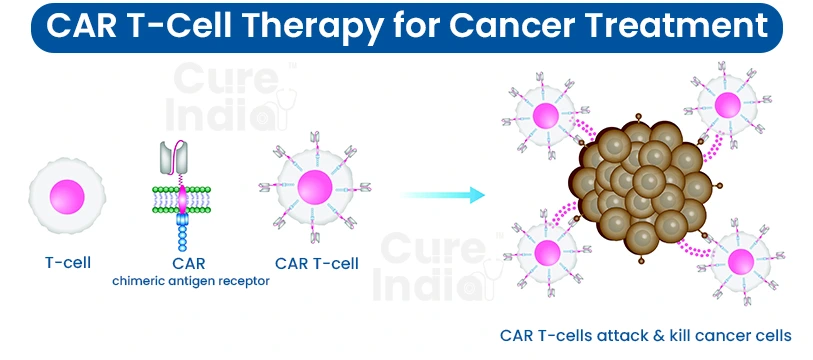
The process is initiated by separating a specific type of white blood cell called T-cells from the patient's bloodstream. This is generally accomplished by leukapheresis, where the blood is drawn from the patient, and the T cells are separated from the other components.
Once the T cells have been removed, they are delivered to a lab where they are genetically engineered. At this stage, scientists transfer a tailored gene into the T cells. This gene codes for a protein called a chimeric antigen receptor (CAR).
The T-cells, which are genetically modified, now start displaying CARs outside their membranes. These CAR T for multiple myeloma are capable of binding to unique proteins on the surface of the cancerous cells.
After the T cells have been modified to express CARs, they are cultured in the laboratory to stimulate their division. This is one of the crucial steps that ensure the effective production of a sufficient number of CAR T multiple myeloma cells for treatment.
When a requisite amount of CAR T cells is obtained, they are set aside to be returned to the patient via infusion. This procedure includes the verification that the cells are of high quality and not contaminated.
The patient can first undergo a preparatory therapy called lymphodepletion before infusion with the CAR T cells. This involves chemotherapy drugs, which aim to reduce the number of existing immune cells in the patient's body. Lymphodepletion makes the infused CAR T cells proliferate and function effectively.
These engineered chimeric antigen receptor T cells are subsequently injected into the patient's body using an IV line. After the CAR T cells are infused, they travel all over the body, looking for cancer cells to attack.
When the CAR T cells recognise the cancer cells that express the specific antigen targeted by the CARs, they bind to these cells and unleash the mechanism that ultimately contributes to their demise. This may involve detaching the toxic substances or initiating other immune cells to fight out the cancer cells.
Some of the infused CAR T cells remain in the patient's body after treatment and act as a long-term surveillance against cancer recurrence. Plus, the continued existence of memory CAR T cells in the background is possible, waiting to attack any future recurrence of cancer.
CAR T-cell treatment can provide a cure for some people who are afflicted with certain blood cancers. The age and the type of cancer determine the eligibility for this treatment:
Patients with some hematologic cancers, such as diffuse large B-cell lymphoma (DLBCL), the primary mediastinal large B-cell lymphoma (PMBCL), and mantle cell lymphoma (MCL), may be treated with CAR T-cell therapy. However, patients may get access to it only if their cancer has not responded to other treatment options or has returned following prior treatment.
CAR T cell therapy is mainly used for leukaemia in children and youth, and it is called B-cell acute lymphoblastic leukemia (B-ALL).
India has come a long way in cancer treatment with its new CAR T-cell therapy called NexCAR19. As such, this therapy is highly disruptive because CAR T treatment can be astronomically expensive, but NexCAR19 is budget-friendly.
It passed the test in October of 2023. In these tests, many patients with advanced leukemia or lymphoma got their cancer size reduced a lot, and some even got utterly better.
Here's how it works: doctors remove T-cells from a patient's immune system and alter them in a lab, turning them into cancer-fighting cells, and then infuse them back into the patient's body. These modified T cells go through fighting cancer.
This is big news for India. NexCAR19 enables many desperate cancer patients who previously lacked the financial ability to undergo treatment. CAR T treatment cost is much less and easily affordable. Along with NexCAR19, other Indian companies also pursue the same lines of research. This implies more chances for cancer treatment in the future.
With CureIndia, you can connect with the best doctors for your CAR T-cell cancer therapy in India. The experienced oncologists and haematologists at CureIndia are experts in administering CAR T-cell therapy, a treatment that uses your immune cells to target and fight cancer. Let us hear from the most experienced CAR T-cell cancer therapy specialists in India:
Dr. Rahul Bhargava is one of India’s leading specialists in CAR T-Cell therapy and cellular immunotherapy. With over 15 years of extensive experience in treating leukemia, lymphoma, and other hematologic cancers, he has helped in bringing advanced cell and gene therapies to India. Dr. Bhargava is highly regarded for his work in bone marrow transplantation and personalised cancer treatments.

Dr. Gaurav Dixit is a highly trained hematologist and oncologist specialising in CAR T-cell therapy, leukemia, and lymphoma management in India. He has experience of around ten years in novel immunotherapies and targeted cancer treatments, particularly in improving outcomes in refractory and relapsed blood cancers.

Dr. Harit Chaturvedi is a senior oncologist with over 35 years of experience in performing advanced cancer therapies, including CAR T-Cell therapy. He leads multidisciplinary oncology programmes that combine advanced immunotherapies with conventional cancer treatments for the best results. He has helped built MICC DNG, one of India's largest oncology programmes.

Dr. Ashok Kumar Vaid is a leading oncologist who is recognised for his expertise in immunotherapy and CAR T-Cell therapy. With around 30 years of experience in managing complex hematological and solid malignancies, he has helped introduce advanced cellular therapies in India.

CAR‑T cell therapy is an advanced and effective treatment option for certain blood cancers, which is available in India at a lower cost than in many Western countries. While prices abroad can exceed $500,000, the cost in India ranges around $85,000, thanks to the development of indigenous therapies like NexCAR19.
| Treatment Name | Cost in India | Stay in India |
|---|---|---|
| CAR T-Cell Therapy in India | $85,000 | 10-15 Days |
The degree of recovery after treatment with CAR T cells differs from one patient to another. Patients might experience the side effects of the therapy right after the session, such as fever, fatigue, or neurological symptoms. Some side effects can be severe and may even require hospitalisation.
After being released from the hospital, patients require close follow-up for any possible complications. We cannot stress enough the importance of having a caregiver around to provide support and to try to stay away from strenuous activities while recovering. Frequent follow-up appointments with healthcare providers are required to track the progress and to handle any long-term side effects.
The time spent on CAR-T cancer treatment in India is affected by different factors, including the type of treatment protocol, the response of the patient to treatment, and the necessity of follow-up care. In general, it may take several weeks for patients to go through the whole CAR-T procedure, such as leukapheresis, cell modification, chemotherapy (if necessary), infusion of CAR-T cells, and initial monitoring of recovery.
